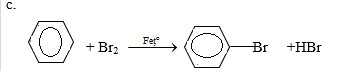
Bài 3 trang 172 SGK Hóa học 11
Lời giải
Bài Tập và lời giải
Vì sao giai cấp tư sản đứng lên chống lại giai cấp quý tộc phong kiến?
Vì sao giai cấp tư sản đứng lên chống lại giai cấp quý tộc phong kiến?
Qua các tác phẩm của mình, các tác giả thời Phục hưng muốn nói lên điều gì ?
Qua các tác phẩm của mình, các tác giả thời Phục hưng muốn nói lên điều gì?
Vì sao xuất hiện phong trào cải cách tôn giáo?
Vì sao xuất hiện phong trào Cải cách tôn giáo?
Em hãy nêu nội dung tư tưởng cải cách của Lu-thơ và Can-vanh.
Em hãy nêu nội dung tư tưởng cải cách của Lu - thơ và Can - vanh.
Nguyên nhân xuất hiện phong trào Văn hóa Phục hưng. Nội dung tư tưởng của phong trào Văn hóa Phục hưng là gì?
Đề bài
Nguyên nhân xuất hiện phong trào Văn hóa Phục hưng. Nội dung tư tưởng của phong trào Văn hóa Phục hưng là gì?
Phong trào Cải cách tôn giáo đã có tác động trực tiếp như thế nào đến xã hội châu Âu thời bấy giờ ?
Phong trào Cải cách tôn giáo đã có tác động trực tiếp như thế nào đến xã hội châu Âu thời bấy giờ?